Neurobiological Insights New Findings on Brain Structure Changes in Schizophrenia
Neurobiological Insights New Findings on Brain Structure Changes in Schizophrenia - Reduced Gray Matter Volume in Key Brain Regions
Reduced gray matter volume in specific brain regions is a prominent feature of schizophrenia. While this is a well-established finding, recent advancements in neuroimaging are offering new details about these structural changes. The frontal and temporal lobes, hippocampus, and orbitofrontal cortex consistently demonstrate reduced gray matter volume in people with schizophrenia. It's not just the amount of gray matter that's altered, but the structure itself. Abnormalities in gyrification, the folds in the brain, further point to disruptions in brain development. These alterations have implications beyond simply being a structural change. They are linked to functional deficits and might contribute to the challenges some people with schizophrenia experience with antipsychotic treatment. The hope is that this deeper understanding of gray matter changes will lead to more targeted and effective diagnostic and therapeutic approaches for this complex disorder.
The shrinking gray matter in certain brain regions is a hallmark of schizophrenia, and it's something that researchers are intensely curious about. We know that the prefrontal cortex and temporal lobes, crucial for executive functioning and auditory processing respectively, are particularly affected. This isn't a uniform change across all patients though - some individuals experience more drastic reductions than others, raising questions about how this might be related to specific symptom patterns or cognitive impairments.
The interesting thing is that these brain changes can appear very early, even before any symptoms are obvious. This suggests that schizophrenia might be influenced by neurodevelopmental factors from the very beginning. It's worth noting that some studies point towards antipsychotics potentially contributing to further gray matter loss over time, which presents a challenging dilemma. The question is whether the benefits of these medications outweigh the potential long-term effects on brain structure.
It's not just about the gray matter though; changes in white matter integrity, which affects communication between different brain regions, also contribute to the cognitive difficulties experienced by individuals with schizophrenia. While this might seem like a bleak picture, the brain is remarkably adaptable and there is evidence that increased connectivity could be a form of compensation for these structural changes.
By using advanced imaging technologies, we can better visualize these subtle changes in brain structure and learn more about their dynamic nature over time. This could have huge implications for improving diagnostic accuracy and guiding treatment strategies for schizophrenia. Intriguingly, the pattern of gray matter reduction we see in schizophrenia resembles that found in other neurodegenerative disorders. This opens up a fascinating avenue of research, where we could explore potential shared mechanisms and explore cross-disciplinary approaches to treatment and prevention.
Neurobiological Insights New Findings on Brain Structure Changes in Schizophrenia - MRI Studies Reveal Consistent Structural Changes
MRI studies have consistently shown that the brains of people with schizophrenia have structural differences compared to healthy people. This is a well-established fact, but researchers are learning more about these changes thanks to advancements in imaging technology. For example, studies have shown that people with schizophrenia have reduced gray matter volume in important brain areas like the prefrontal cortex and the temporal lobes. These differences aren't uniform, though. The amount of gray matter loss varies significantly between individuals. This makes things more complicated for researchers trying to understand how these changes contribute to the symptoms and cognitive challenges experienced by people with schizophrenia.
Looking at brain structure over time is also important. Longitudinal studies help us see how these changes develop and how they might impact treatment outcomes. It's important to remember that schizophrenia is a complex disorder and these brain differences are just one piece of the puzzle. However, understanding these structural changes is critical, as it could lead to more targeted treatments and potentially even better ways to diagnose schizophrenia in the future.
Over 500 MRI studies, involving over 38,000 individuals, have been conducted to compare brain structures between people with schizophrenia and healthy controls. These studies have consistently shown that the brains of individuals with schizophrenia exhibit reduced gray matter volume, particularly in the prefrontal, medial, and superior temporal lobes. This reduction is often more pronounced in individuals who transition from preclinical stages to psychosis. While we've known for a while about these changes, the sheer volume of research using MRI has allowed us to get a much clearer picture of how these structural differences manifest at different stages of the disorder.
It's important to note that the pattern of gray matter loss isn't uniform across all individuals with schizophrenia. This variation in brain structure has led researchers to consider how different brain alterations might correlate with varying symptom presentations and clinical outcomes. We're starting to see that the structural changes in schizophrenia are not simply isolated events, but part of a dynamic process that can be observed over time.
These changes can happen early in life, suggesting that neurodevelopmental factors play a significant role in the onset of schizophrenia. Longitudinal MRI studies have allowed us to track changes in brain structure over time, providing invaluable insights into the progression of the disease. This research is vital in understanding the potential role of early interventions and exploring the possible impact of antipsychotic medications on brain structure. While we understand the link between these structural alterations and cognitive deficits, we need to continue studying how they interact with each other and how they might be influenced by treatment. Ultimately, this research is laying the foundation for a more targeted and individualized approach to treating this complex and multifaceted disorder.
Neurobiological Insights New Findings on Brain Structure Changes in Schizophrenia - Stronger Genetic Covariation with Brain Structure
The relationship between genetic factors and brain structure in schizophrenia is becoming increasingly complex. New research suggests that the genetic influence on brain structure is especially strong in key areas of the brain, often called "hubs" of structural covariance networks. These hubs play a critical role in how different brain regions communicate and function together. This discovery points to specific regions on chromosomes that could be involved in shaping the development of these crucial brain structures.
There's also a strong correlation between the genetic similarities between different areas of the cortex and how those areas behave. This means that genetic factors strongly influence how different brain regions interact, especially in individuals who are genetically predisposed to schizophrenia. Understanding how genes and brain structure interact is vital, as it might provide valuable insights into the development of schizophrenia and potentially lead to improved diagnosis and treatment strategies.
The genetic basis of brain structure in schizophrenia is becoming clearer, but it's still a complex puzzle. Recent research suggests that genetic variations play a significant role in the brain changes we see in people with schizophrenia. It seems like some specific genes might be linked to abnormalities in certain brain areas. The intriguing thing is that there might be shared genetic pathways between schizophrenia and neurodegenerative diseases, implying common biological mechanisms at play.
We're learning that some brain volume changes, especially in gray matter, are influenced by genetics and can be inherited. This means that individuals with a family history of schizophrenia may be at higher risk for specific structural brain changes. However, not everyone with schizophrenia experiences the same brain changes, and this variability is linked to differences in their genetic makeup. This reinforces the fact that schizophrenia is not a one-size-fits-all disorder and that understanding its causes requires a personalized approach.
The relationship between genetics and brain structure seems to change over time, suggesting that the interplay between genetic factors and brain development is dynamic. This dynamic aspect could reveal new insights into how and when genetic risk influences brain development.
With advanced neuroimaging, we're able to link genetic profiles with brain structure changes, offering a much richer understanding of how genetic variations translate into visible brain differences. This connection has implications not only for understanding schizophrenia's development but also for its cognitive deficits. The relationship between genetics and brain structure highlights the importance of studying how these genetic factors may influence cognitive function.
While genetic covariation is significant, environmental factors like stress may interact with genetic predispositions to exacerbate brain structural changes. Understanding this interaction is critical for developing comprehensive treatment strategies. The strong correlation between certain genetic markers and specific brain structural changes might lead to the identification of biomarkers for early detection and diagnosis of schizophrenia, opening the door for timely interventions.
The field is moving forward with new technologies like gene editing and advanced neuroimaging, which will help us unravel the intricate relationship between biology, brain structure, and function. The hope is that this research will lead to new therapeutic approaches for schizophrenia. It's a complex and fascinating area of study, and the journey towards understanding the genetic basis of schizophrenia is just beginning.
Neurobiological Insights New Findings on Brain Structure Changes in Schizophrenia - Advances in Understanding Brain Cellular Composition
Recent advances in our understanding of the brain's cellular makeup have significant implications for mental health research, particularly in relation to schizophrenia. New tools like single-cell RNA sequencing and spatial transcriptomics are providing us with unprecedented detail about the various types of brain cells and how they function during development and disease. These innovations have revealed specific genetic influences on brain cell composition that seem to correlate with an increased risk for schizophrenia. This opens up exciting new possibilities for developing individualized therapies that target specific cell types. It is also increasingly clear that human brain evolution involves unique genomic and molecular mechanisms compared to other species. This highlights the complex challenges associated with understanding and treating schizophrenia. As research progresses, these insights have the potential to dramatically improve our ability to detect schizophrenia early, diagnose it more accurately, and develop more effective treatments.
The brain is a remarkably complex organ, and its cellular composition is fundamental to understanding its function and dysfunction. While our understanding of the brain's cellular makeup has evolved significantly over the past 50 years, recent breakthroughs in research are challenging existing notions. Advancements in single-cell RNA sequencing are revealing not just the types of cells in the brain, but also their unique gene expression profiles. This level of detail is crucial for understanding the diverse cellular landscape of the brain and how it might contribute to the development of disorders like schizophrenia.
For instance, while we know the brain contains approximately 86 billion neurons, it actually houses an even greater number of glial cells—three times as many, in fact. This highlights the potential importance of glial cells in mental health disorders, previously overshadowed by the focus on neuronal activity. Interestingly, recent research suggests that specific subtypes of inhibitory interneurons, particularly those expressing parvalbumin, may be crucial for resilience to schizophrenia symptoms. This raises intriguing questions about why certain cell populations can maintain functionality under stress, while others falter.
Emerging imaging studies also point to alterations in the distribution of specific neurotransmitter receptors, like the NMDA receptor, in the brains of individuals with schizophrenia. This offers potential targets for precision medicine approaches, which are designed to address the unique needs of individual patients based on their specific biology.
Moreover, the altered neuroinflammatory responses observed in schizophrenia seem to stem from a unique shift in the brain's cellular composition. This shift involves specific immune cells, like microglia, exhibiting both overactivity and dysfunction, further complicating our understanding of the disorder's origins. What's even more fascinating is that these cellular changes don't seem to be confined to regions traditionally associated with schizophrenia, like the prefrontal cortex. They extend to subcortical structures, including the amygdala and hippocampus, demonstrating the disorder's widespread impact on brain function.
Researchers are also investigating the hypothesis that neurodevelopmental changes leading to schizophrenia might stem from altered cellular signaling pathways during critical periods of brain maturation. This could explain why some individuals develop symptoms earlier in life. Studies are also indicating that the cellular composition of the brain can vary not only between individuals diagnosed with schizophrenia, but also among those at high genetic risk for the disorder. This suggests that cellular markers may hold promise for identifying individuals who might be at risk for schizophrenia before the onset of clinical symptoms.
The concept of brain plasticity in relation to schizophrenia is also being re-examined. Recent findings reveal that certain cellular changes may be reversible or compensable with targeted interventions, highlighting the potential for novel therapeutic approaches.
The gut-brain axis is another exciting area of research. Scientists are exploring the possibility that cellular composition in the gut might influence neuroinflammatory states, ultimately impacting brain structure and function. This intriguing connection opens a new avenue for understanding the diagnosis and treatment of schizophrenia. The continued exploration of the brain's cellular composition promises to provide crucial insights into the causes and potential treatments for schizophrenia.
Neurobiological Insights New Findings on Brain Structure Changes in Schizophrenia - Spatial Correlates Linked to Clinical Symptom Profiles
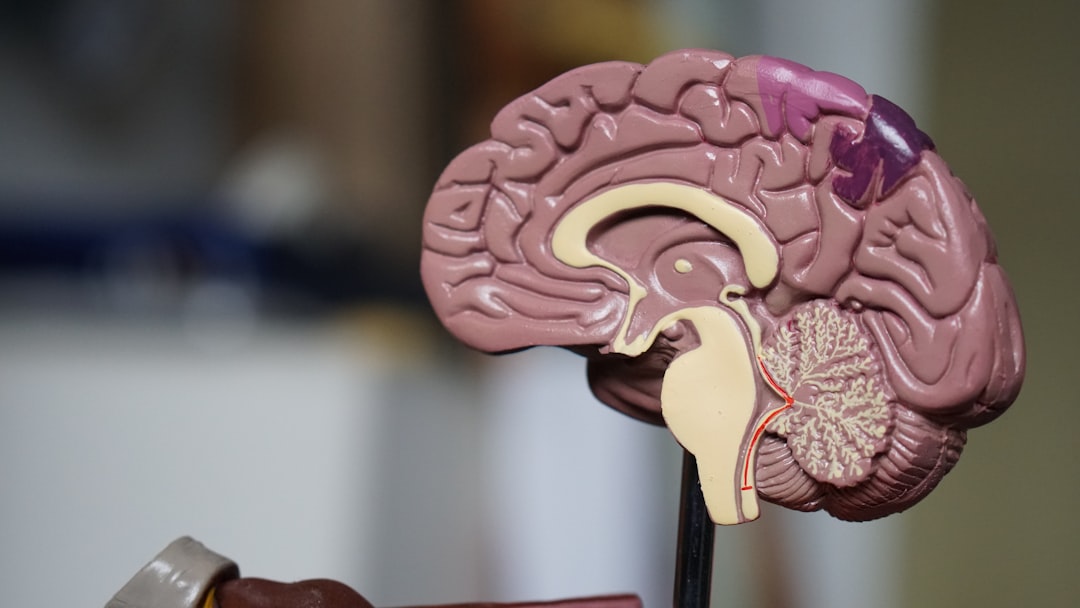
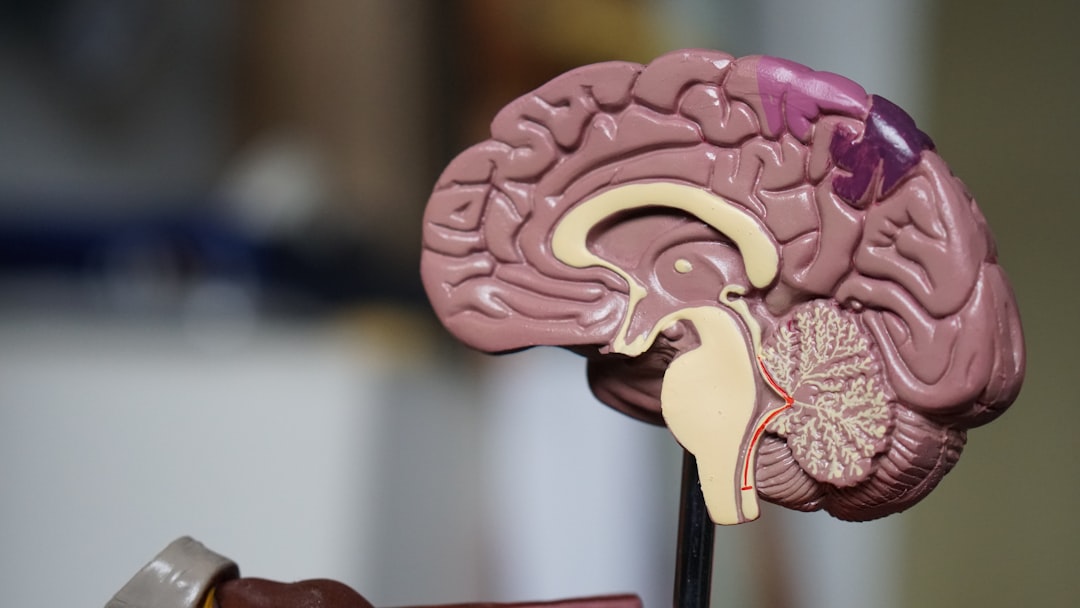
The way specific brain structures are linked to the different symptoms of schizophrenia is becoming clearer. Scientists have used brain scans to show that certain parts of the brain have unique changes in structure and function based on the type of symptoms a person is experiencing. This means that certain symptoms, like hallucinations or problems with thinking, might be caused by changes in particular brain regions. The cerebellum, the part of the brain usually known for movement, has been found to play a bigger role in schizophrenia than previously thought.
Interestingly, the amount of gray matter and how well the white matter is working in the brain seems to be tied to the severity of both positive (things like hallucinations) and negative (things like lack of motivation) symptoms. This hints that the disorder might be caused by problems in how different parts of the brain communicate with each other. The research is showing that these brain changes could be useful in creating more individualized treatments that target the specific parts of the brain that are affected in each person. This highlights how closely connected a person's brain structure is to the specific symptoms they experience.
The search for the root causes of schizophrenia is a complex and multi-layered endeavor. While reduced gray matter volume in key brain regions is well established, we're diving deeper into understanding how specific structural changes correlate with different symptom profiles.
It's intriguing to see how abnormalities within the frontal and temporal lobes – areas crucial for executive functioning and auditory processing – are directly tied to clinical presentations. The hippocampus, essential for memory and learning, also shows significant gray matter reductions, which might explain the cognitive deficits and distorted thought patterns frequently seen in patients.
We're also examining gyrification—the patterns of folds in the brain's surface. It's no longer simply a matter of anatomical observation. These changes could signify disruptions in brain development, contributing to the onset and severity of schizophrenia.
But here's a crucial point: Gray matter loss isn't uniform. It varies significantly between individuals, emphasizing the need for personalized treatments. Imagine tailoring strategies to the unique structural changes within a person's brain, leading to more effective management.
Even more intriguing are findings from MRI studies that reveal structural brain changes years before clinical symptoms manifest. This could pave the way for early diagnosis and preventive measures, particularly within at-risk populations.
Further research explores the communication networks within the brain, particularly the weakening interconnectivity between cerebral regions during psychotic episodes. Could strengthening these connections, especially in the prefrontal cortex, be a key to slowing or even mitigating the progression of schizophrenia?
However, there's a difficult question arising from longitudinal studies: Some antipsychotic medications, while effectively managing symptoms, appear to contribute to further gray matter reduction over time. The ongoing debate is whether the benefits of these medications outweigh the potential long-term effects on brain structure.
Adding another layer of complexity, spatial analyses of immune cells within the brain reveal that altered neuroinflammatory responses might contribute to brain structure changes in schizophrenia. This suggests that inflammation could be a central element in the disorder's pathology.
The interplay between genetics and structure is also becoming clearer. Specific genetic variations, particularly within "hubs" of structural covariance networks, seem to be linked to specific brain regions affected by schizophrenia. This is a potential avenue for genetic testing in risk assessment, opening up new possibilities for early intervention.
But the story doesn't stop there. The impact of schizophrenia extends beyond the prefrontal cortex. We're seeing structural alterations in subcortical regions like the amygdala and thalamus. It seems that the disorder disrupts the entire architecture of the brain, not just isolated areas.
This is just a glimpse into the ongoing research. We're unraveling the complex relationships between structure, function, and genetics, and it's a journey that promises to reveal new and more targeted approaches to treating this challenging disorder.
More Posts from psychprofile.io:
- →Unveiling the Subtle Signs 7 Lesser-Known Symptoms of Adult ADHD
- →Alarming Trends Analyzing the 36% Increase in US Suicide Rates from 2000 to 2022
- →Understanding First-Episode Psychosis Early Warning Signs and Neurobiological Markers in Young Adults
- →Exploring Teen Bipolar Disorder Through AI Profiling
- →Neurobiological Markers of Severe PTSD Latest Research Findings
- →Exploring AI Techniques for Depression Symptom Detection